Para-Equilibrium
Pandat provides a function to calculate para-equilibrium. The detail description and definition of para-equilibrium can be found in the book of Professor Hillert [1998Hil].
In a normal phase equilibrium, all components are considered to be mobile, freely to move from one phase to another to reach the equilibrium state, in which the same component in different phases has identical chemical potential.
The concept of para-equilibrium applies to alloy systems where there is a large difference in mobility of the different components. Then some components can be treated as mobile and others as immobile. In a para-equilibrium, the ratio of molar fractions of immobile components in one phase is the same as that in other phases:
|
|
However, the chemical potential of an immobile component in one phase is different from that in other phases. In rare cases an immobile component may have the same chemical potential in different phases. The same mobile component in different phases in para-equilibrium has the same chemical potential: (m: a mobile component)
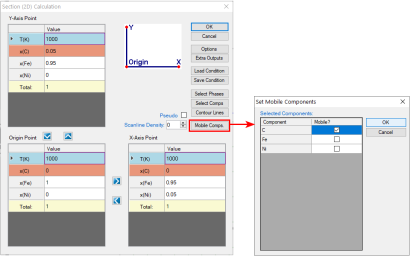
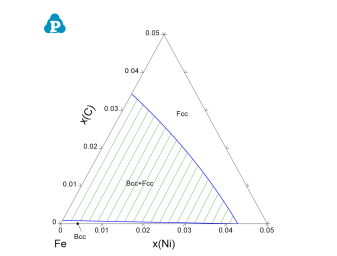
The calculation for a para-equilibrium follows the same procedure as the normal calculation except for setting the mobile components before calculation. In dialog window for all three calculations of Point (0D), Line (1D), and Section (2D), there is a button “Mobile Comps.” After clicking on this button, a new dialog window pops up for setting the mobile components. As shown below are the dialog windows for setting an isothermal section calculation for Fe-Ni-C system with C is mobile. The default state is that the all the components are immobile. When the element is set as “mobile comps”, After click “OK”, the mobile component will be shown as orange at the calculation condition setting interface, as shown in Figure 1. The calculated para-equilibrium isothermal section is shown in Figure 2.
[1998Hil] M. Hillert. Phase Equilibria, “Phase Diagrams and Phase Transformations: Their Thermodynamic Basis”. United Kingdom: Cambridge University Press, 1998.