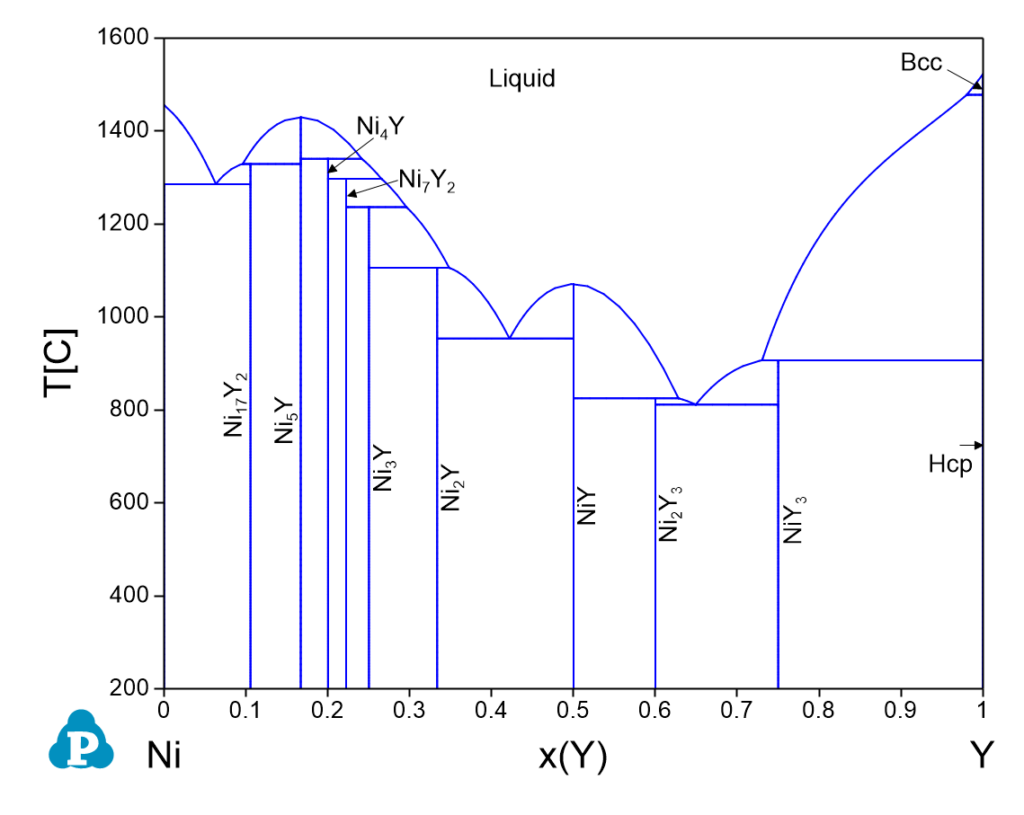
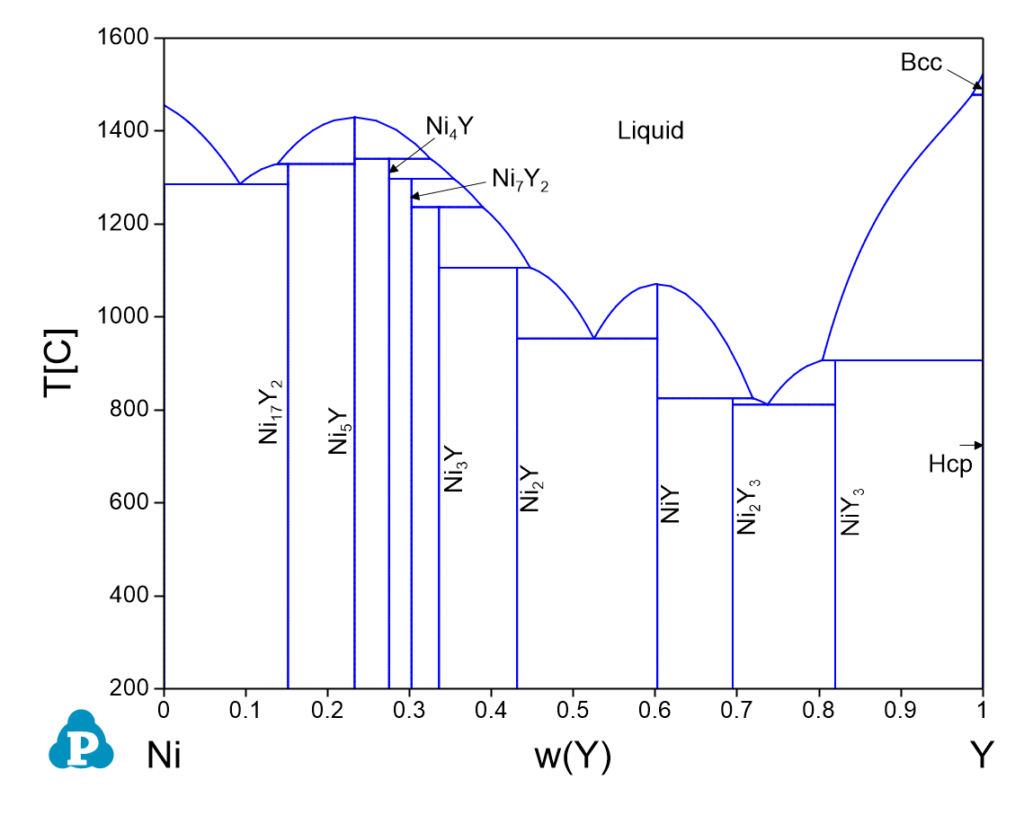
Ni-Y Phase Diagrams
Invariant React
| T | reaction | x(Ni) | x(Y) | w(Ni) | w(Y) |
| C | mole/mole | mole/mole | kg/kg | kg/kg | |
| 1478.0 | Bcc => Liquid + Hcp | 0.0195 | 0.9805 | 0.0130 | 0.9870 |
| 1340.3 | Liquid + Ni5Y => Ni4Y | 0.7589 | 0.2411 | 0.6751 | 0.3249 |
| 1329.1 | Liquid + Ni5Y => Ni17Y2 | 0.9041 | 0.0959 | 0.8616 | 0.1384 |
| 1297.2 | Liquid + Ni4Y => Ni7Y2 | 0.7348 | 0.2652 | 0.6465 | 0.3535 |
| 1285.7 | Liquid => Fcc + Ni17Y2 | 0.9369 | 0.0631 | 0.9075 | 0.0925 |
| 1236.4 | Liquid + Ni7Y2 => Ni3Y | 0.7043 | 0.2957 | 0.6113 | 0.3887 |
| 1105.6 | Liquid + Ni3Y => Ni2Y | 0.6520 | 0.3480 | 0.5529 | 0.4471 |
| 953.4 | Liquid => Ni2Y + NiY | 0.5782 | 0.4218 | 0.4750 | 0.5250 |
| 906.8 | Liquid + Hcp => NiY3 | 0.2696 | 0.7304 | 0.1960 | 0.8040 |
| 825.2 | Liquid + NiY => Ni2Y3 | 0.3719 | 0.6281 | 0.2811 | 0.7189 |
| 811.6 | Liquid => Ni2Y3 + NiY3 | 0.3506 | 0.6494 | 0.2627 | 0.7373 |