PanPhaseDiagram
PanPhaseDiagram module is for the calculation of phase equilibria and thermodynamic properties of multi-component, multi-phase systems. In addition to the stable and metastable phase equilibria, phase transformation temperature, phase fraction, and thermodynamic properties, such as Gibbs energy, enthalpy, entropy and chemical driving force, can all be readily calculated. This module can also be used for solidification simulation based on Scheil model and Lever rule, and provide latent heat and total heat evolved during solidification.

PanPhaseDiagram
- stable and metastable diagrams
- property contour diagrams
- thermodynamic properties
- chemical driving force
- material to material diagrams
Some Key Features of PanPhaseDiagram Module
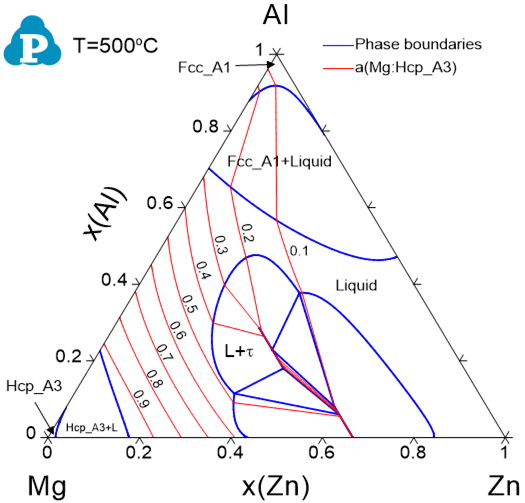
Contour diagram showing the activity of Mg in the Al-Mg-Zn ternary system at 500oC
- Phase diagrams: isotherm, isopleth, phase projection for an n-component system, stability diagram, 3D phase diagram, pseudo-binary section, pressure diagram
- Property contour diagrams: contour diagrams for thermodynamic properties and physical properties, such as activity, phase fraction, density, driving force and surface tension
- Phase equilibria: stable and metastable phase equilibria
- Phase properties: amounts and compositions of phases, phase transformation temperatures
- Solidification: solidification path and heat evolution using the Scheil and Lever-rule models
- Thermochemical properties: enthalpy of formation, activity, partial pressure, partial molar properties, excess properties and driving force
- Thermophysical properties: molar volume, density, surface tension and viscosity
- Special properties: magnetic transition, second order phase transformation, zero-phase-fraction lines, reaction equations, spinodal decomposition curve, electrical and thermal conductivity
- Mobility and diffusivities: self, tracer, chemical and inter-diffusivities

