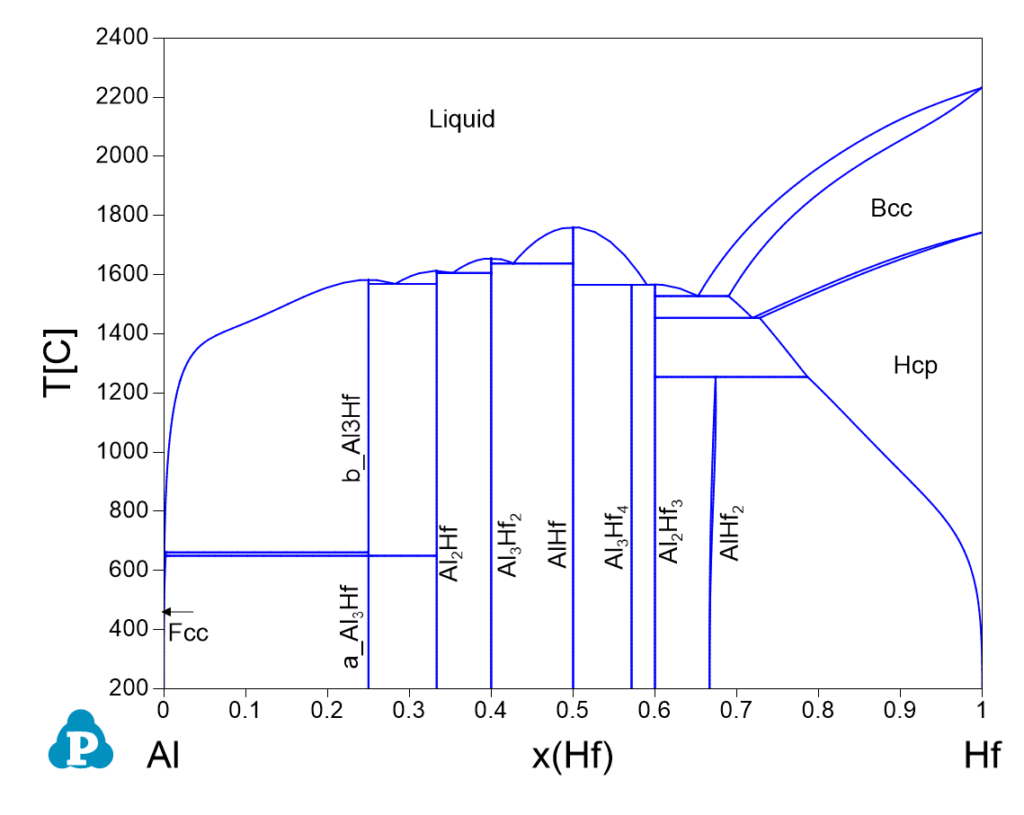
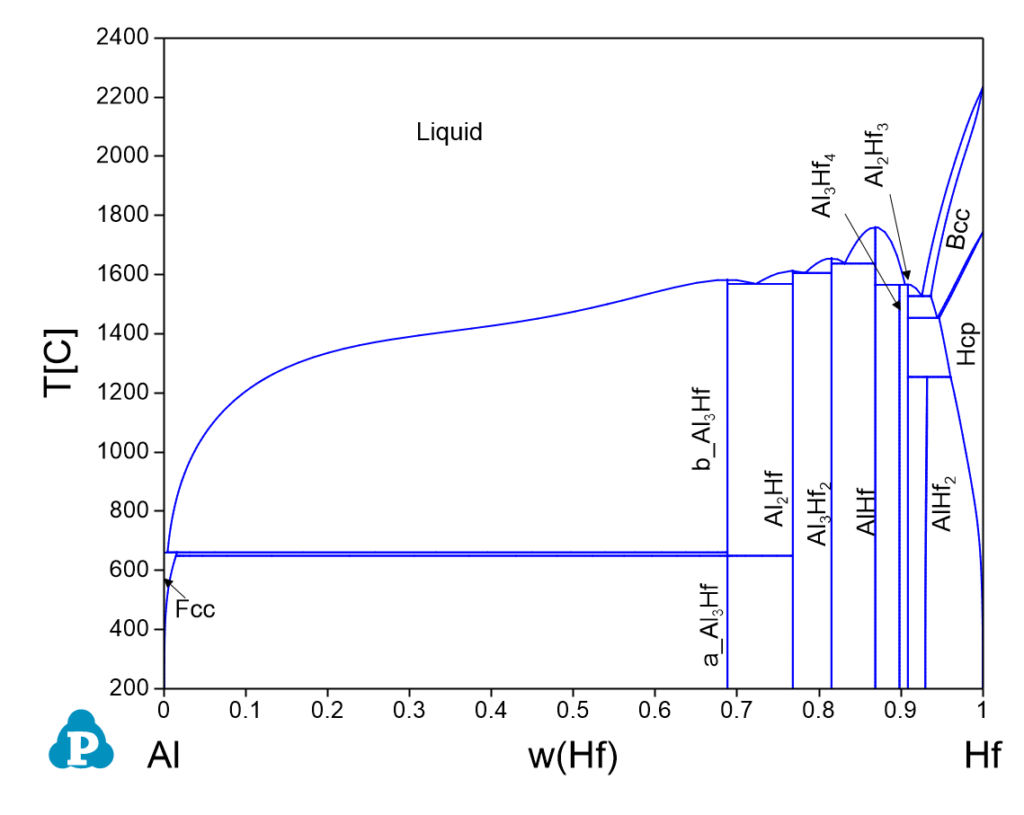
Al-Hf Phase Diagrams
Invariant Reactions
| T | reaction | x(Al) | x(Hf) | w(Al) | w(Hf) |
| C | mole/mole | mole/mole | kg/kg | kg/kg | |
| 1638.0 | Liquid => Al3Hf2 + AlHf | 0.5732 | 0.4268 | 0.1688 | 0.8312 |
| 1605.6 | Liquid => Al2Hf + Al3Hf2 | 0.6470 | 0.3530 | 0.2169 | 0.7831 |
| 1568.4 | Liquid => b_Al3Hf + Al2Hf | 0.7174 | 0.2826 | 0.2773 | 0.7227 |
| 1565.5 | Liquid + AlHf => Al3Hf4 | 0.4098 | 0.5902 | 0.0950 | 0.9050 |
| 1565.4 | Liquid => Al3Hf4 + Al2Hf3 | 0.4286 | 0.5714 | 0.1018 | 0.8982 |
| 1527.4 | Liquid => Al2Hf3 + Bcc | 0.3474 | 0.6526 | 0.0745 | 0.9255 |
| 1453.8 | Bcc => Al2Hf3 + Hcp | 0.2810 | 0.7190 | 0.0558 | 0.9442 |
| 1254.6 | Al2Hf3 + Hcp => AlHf2 | 0.2136 | 0.7864 | 0.0394 | 0.9606 |
| 661.6 | Liquid + b_Al3Hf => Fcc | 0.9994 | 0.0006 | 0.9960 | 0.0040 |
| 649.9 | Al2Hf + b_Al3Hf => a_Al3Hf | 0.7500 | 0.2500 | 0.3120 | 0.6880 |
| 649.9 | b_Al3Hf => Fcc + a_Al3Hf | 0.9978 | 0.0022 | 0.9857 | 0.0143 |