PanRHEA
Database for refractory high entropy alloys (RHEA)
- Thermodynamic
- Mobility
- Thermophysical Property: Molar Volume; Surface Tension; Viscosity
PanRHEA
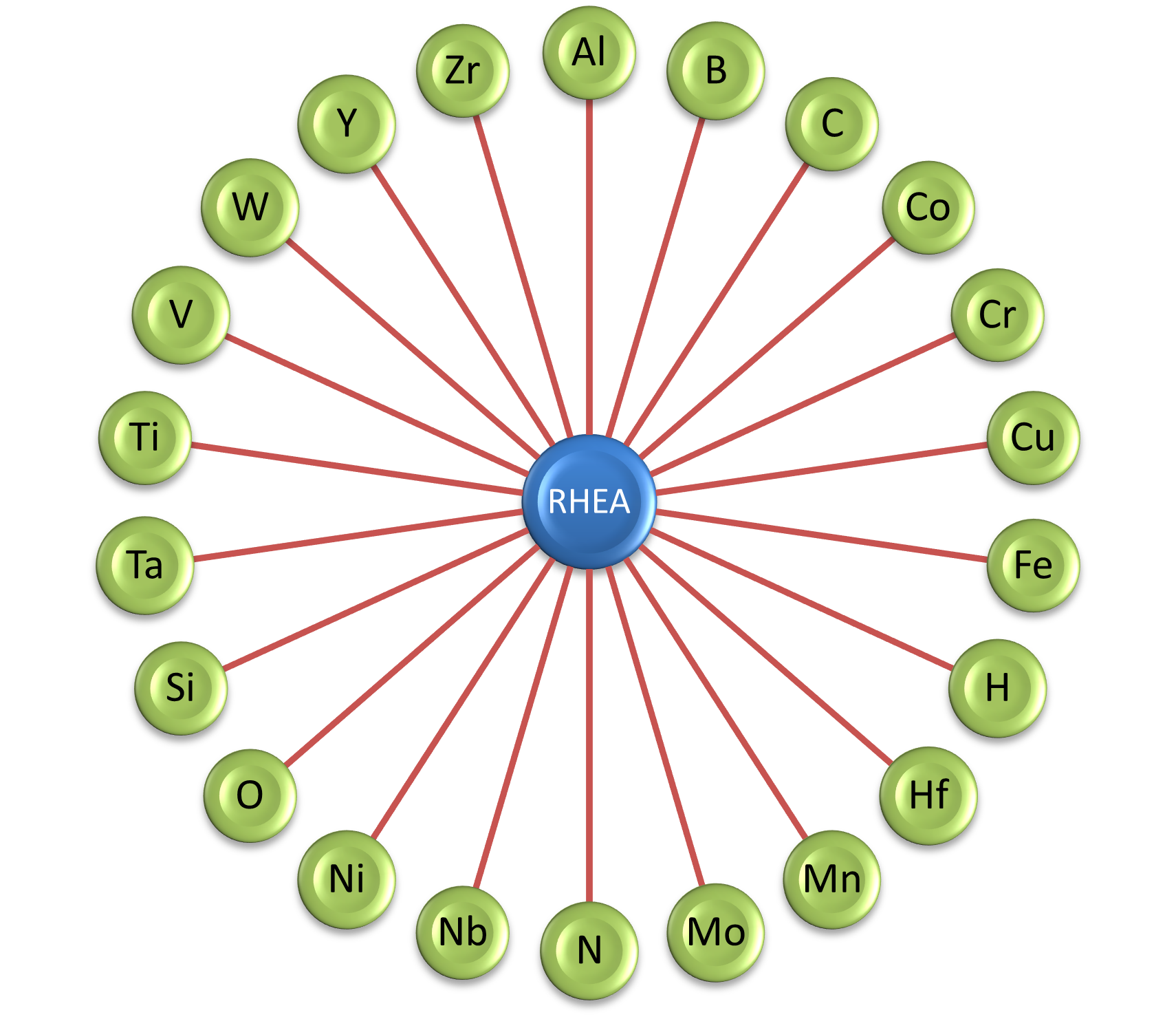
PanRHEA Quick Overview
-
Number of components: 23
-
Number of phases: 524
-
Major alloying elements: Al, Co, Cr, Cu, Fe, Hf, Mn, Mo, Nb, Ni, Ru, Si, Ta, Ti, V, W, Y and Zr
-
Minor alloying elements: B, C, H, N and O
PanRHEA – Thermodynamic database (TH), Mobility database (MB), and Thermophysical property (TP) database for refractory high entropy alloys (RHEA).
- The current PanRHEA_TH thermodynamic database consists of 23 components, 524 phases. 238 binaries and 223 constituent ternaries have been critically assessed.
- The current PanRHEA_MB mobility database includes assessed mobility model parameters for the Liquid, Bcc, Fcc, and Hcp solution phases. It is compatible with the PanRHEA_TH and suitable for the simulation of diffusion controlled phenomena of multi-component RHEAs.
- The PanRHEA_TP thermophysical property database includes the molar volume (MV) database covers all the phases assessed in the PanRHEA_TH, the surface tension and viscosity properties for the liquid phase. It can be combined with the thermodynamic database for the simulation of thermo-physical properties of RHEA alloys, such as density, thermal expansion, and solidification shrinkage.
Applications
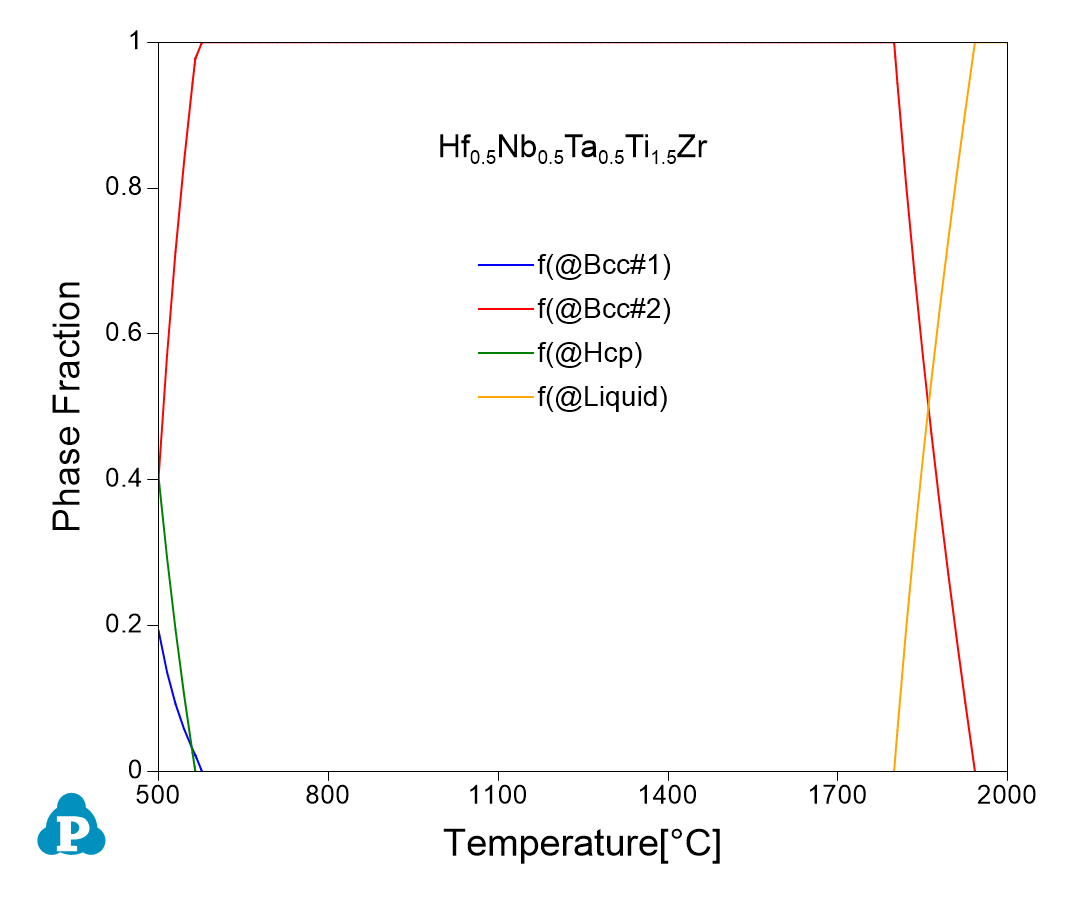
Equilibrium calculation of the Hf0.5Nb0.5Ta0.5Ti1.5Zr RHEA alloy
The calculated phase equilibrium of the Hf0.5Nb0.5Ta0.5Ti1.5Zr vs. temperature using the PanRHEA database shows that the Bcc phase is stable within a large temperature range and three phases (Bcc#1+Bcc#2+Hcp) coexist at 500 °C, which is consistent with the experimental observations well in the literature.
This example shows the simulated concentration profile of Mo-W binary alloys at 2318 K in good agreement with the experimental data.
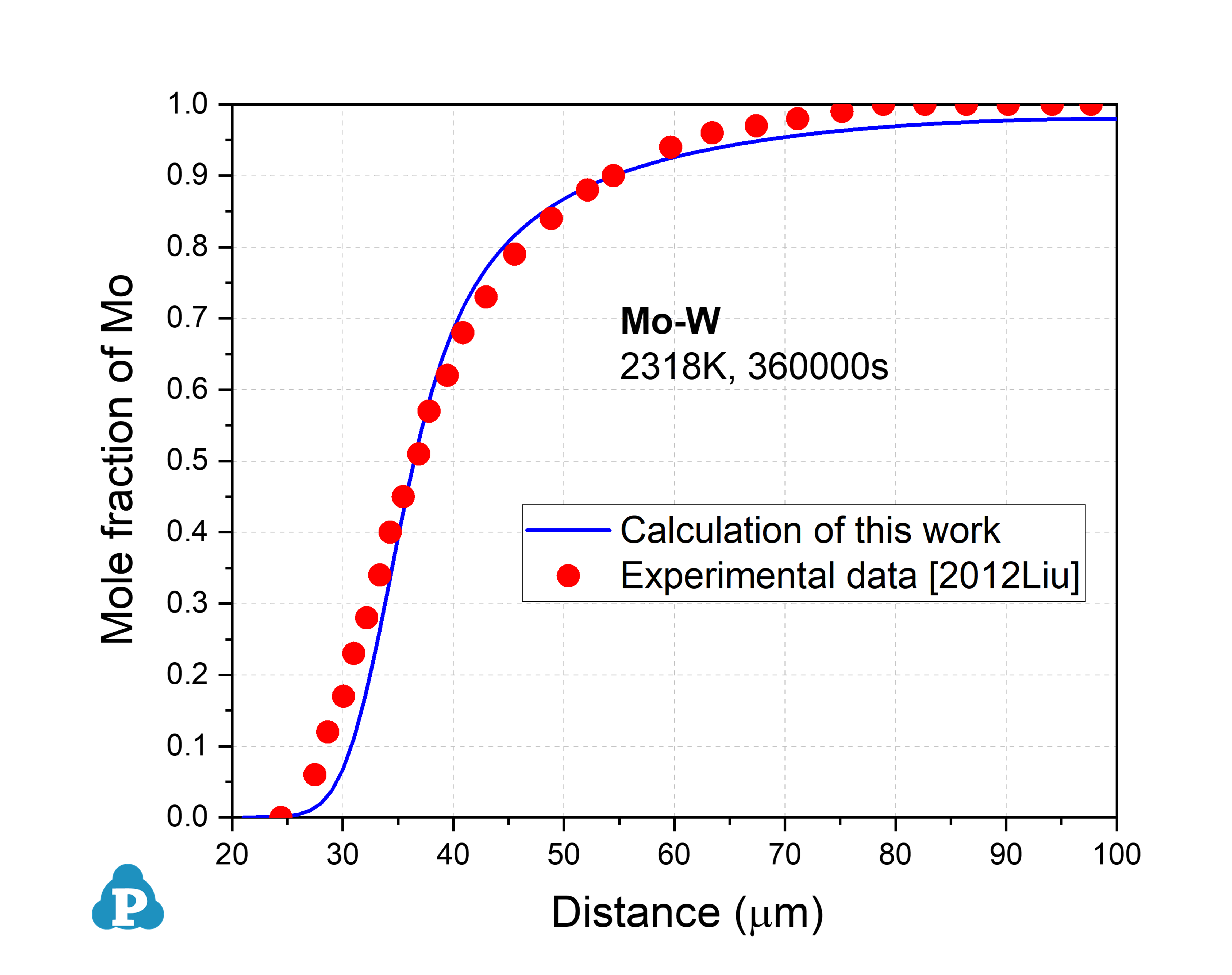
Concentration profiles of Bcc Mo-W at 2318K for 100h
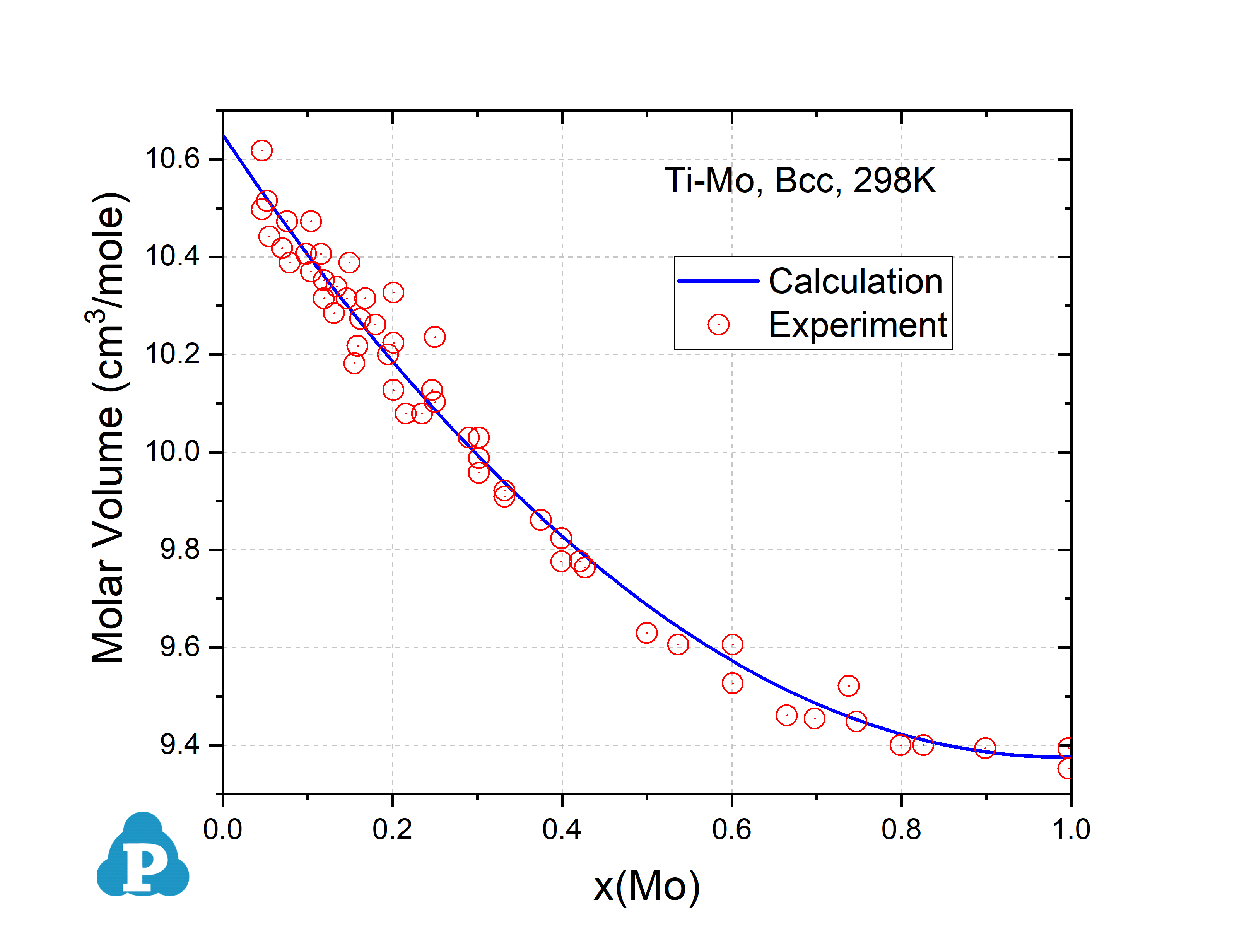
Calculated molar volume of Bcc Mo-Ti at 298 K compared with experimental data
This example shows the calculated molar volume of Bcc Mo-Ti at 298 K in good agreement with the experimental data.

